Mass Formula Chemistry
Watch for rounding errors in the last significant figure to make sure all the percentages add up. Total momentum Total mass X velocity of the center of mass.

Formula Triangle Molarity Teaching Chemistry Chemistry Classroom Chemistry Lessons
The relative atomic mass scale is used to compare the masses of different atoms.

. In chemistry the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula then adding all of these products together. Group intranet Restricted permissions Barrow Group photos Restricted permissions Group calendar Restricted permissions Calculators. This is also known as autoprotolysis or amphoteric nature of water.
The Mass percent formula is expressed as solving for the molar mass also for the mass of every element in 1 mole of the. Different molecules of the same compound may have different molecular masses because they contain different isotopes of an element. Mass percent is also known as percent by weight or ww.
This simply means the calculation is performed using relative atomic weight values for the elements which are based on the natural isotopic ratio of elements found in Earths atmosphere and crust. Given Total momentum 16 kgmseci. It is measured in daltons Da or u.
Because the individual masses of the ingredients of a mixture sum to their mass fractions sum to unity. During this article well learn the mass percent formula with various solved numerical. Mass percent mass of chemicaltotal mass of compound x 100.
Mass percent mass of chemicaltotal mass of compound x 100. The basic formula for mass percent of a compound is. Pure water undergoes auto-ionization or self-ionization by donating or accepting a proton between two molecules of water to form H 3 O and OH ions.
4 Important of Molar Mass. Introduction to the water ionization constant K w. Mass of one object5 kg.
Solve out any chemical reaction using these formulas only from ClearIITMedical. By using this chemists work out the chemical formula. The sum of all the mass percentages should add up to 100.
In chemistry the mass fraction of a substance within a mixture is the ratio alternatively denoted of the mass of that substance to the total mass of the mixture. The units of mass are typically grams. To calculate m and ν.
The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. In chemistry the molar mass is an important quantity. Mass fraction can also be expressed with a denominator of 100 as.
Petroleum and energy links. The molecular mass m is the mass of a given molecule. Textdensity fractextmasstextvolume Lets practice finding volume using mass density with.
A related term you should know is relative formula mass relative formula weight. It has six carbons twelve hydrogens and six oxygens. The molar mass of glucose is the sum of the relative atomic.
One of the objects has a mass 5 kg and velocity 16 msi. 174 convert the given mass of a substance to the amount of the substance in moles and vice versa by using the relative atomic or formula mass. Quantitative chemistry - AQA.
Mass percent components mass total mass x 100 or. Percentage of mass solutes mass mass of solution x 100. Glucose molecular formula is C 6 H 12 O 6.
If the formula used in calculating molar mass is the molecular formula the formula. Expressed as a formula the mass fraction is. Velocity of the center of mass 2 msi.
Velocity of this object16 msi. Write the equation at the beginning of every problem. And is given by the formula.
The related quantity relative molecular mass as defined by IUPAC is the ratio of the mass of a molecule to the unified atomic mass unit also known as. Get free list of Chemistry formulas online at ClearIITMedical. You must multiply by 100 at the end to express the value as a percentage.
It measures the mass of a mole of a given substance. In chemistry the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula then adding all of these products together. The mass and velocity of the other objects.
The relative formula mass of a substance made up of molecules. The molar mass is the sum of the masses of all the atoms in one mole of the compound. If the formula used in calculating molar mass is the molecular formula the formula.
Relative Formula Mass Definition. Is the sum of the relative atomic masses of the atoms in the numbers shown in the formula. Density is the amount of mass per unit volume.

Step 25 Mass Molar Mass And Mole Relationship 100 Steps To Sat Ii Chemistry From Unisprint Sat Satchem Chemistry Lessons Chemistry Ap Chemistry

Calculating Average Atomic Mass Chemistry Lessons Chemistry Classroom Teaching Chemistry
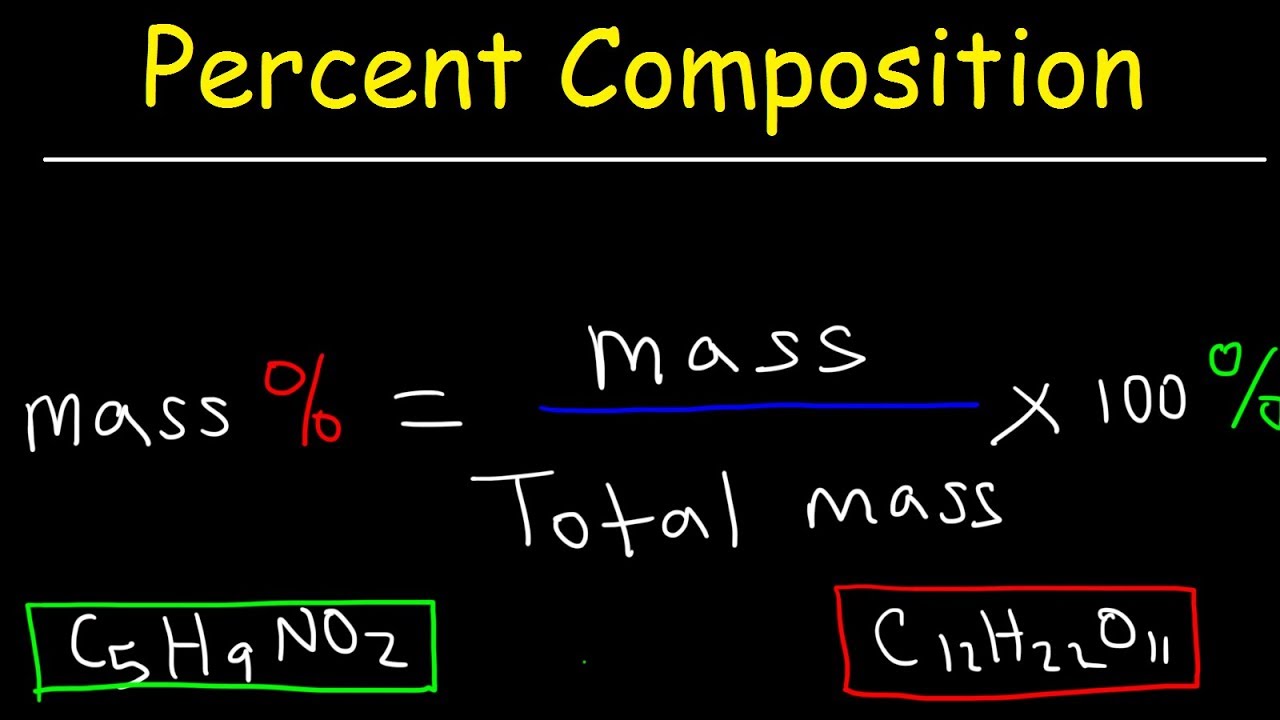
Chemistry Lesson Percent Composition Youtube Chemistry Lessons Teaching Chemistry Ap Chemistry

Difference Between Formula Mass And Molecular Mass Comparison Summary Chemistry Lessons Teaching Chemistry Chemistry Education
0 Response to "Mass Formula Chemistry"
Post a Comment